You are now leaving the ISTURISA (osilodrostat) website. This link will take you to a site maintained by a third party who is solely responsible for its content.
Cushing’s Disease Causes Elevated Cortisol
Cushing’s disease (CD) is a rare hormonal disorder caused by a pituitary adenoma that secretes excess adrenocorticotropic hormone (ACTH).1,2 Excess ACTH stimulates the adrenal glands to overproduce cortisol, leading to the clinical manifestations of CD.1
Prevalence & Impact
Although rare, Cushing’s disease is the most prevalent of all cushing’s syndrome cases.3 And while it’s uncommon, it’s a serious condition:
- Prevalence of nearly 40 cases per million4
- 1.2 to 2.4 new cases/million/year4
- 3x more likely to develop in women, most commonly between ages 30 and 604,5
are caused by an
ACTH-secreting
pituitary tumor
are caused by
primary adrenocortical
tumors
are caused by ectopic
ACTH-secreting
non-pituitary tumors
Comorbidities are common
Cushing’s disease patients experience comorbidities at a higher rate than the general population.1 Although biochemical remission or a cure is usually associated with significant clinical improvement, some comorbidities may not completely normalize.6 Hypertension and diabetes are the main long-term controllable risk factors for cardiovascular events and mortality; repeated follow-up is mandatory.6
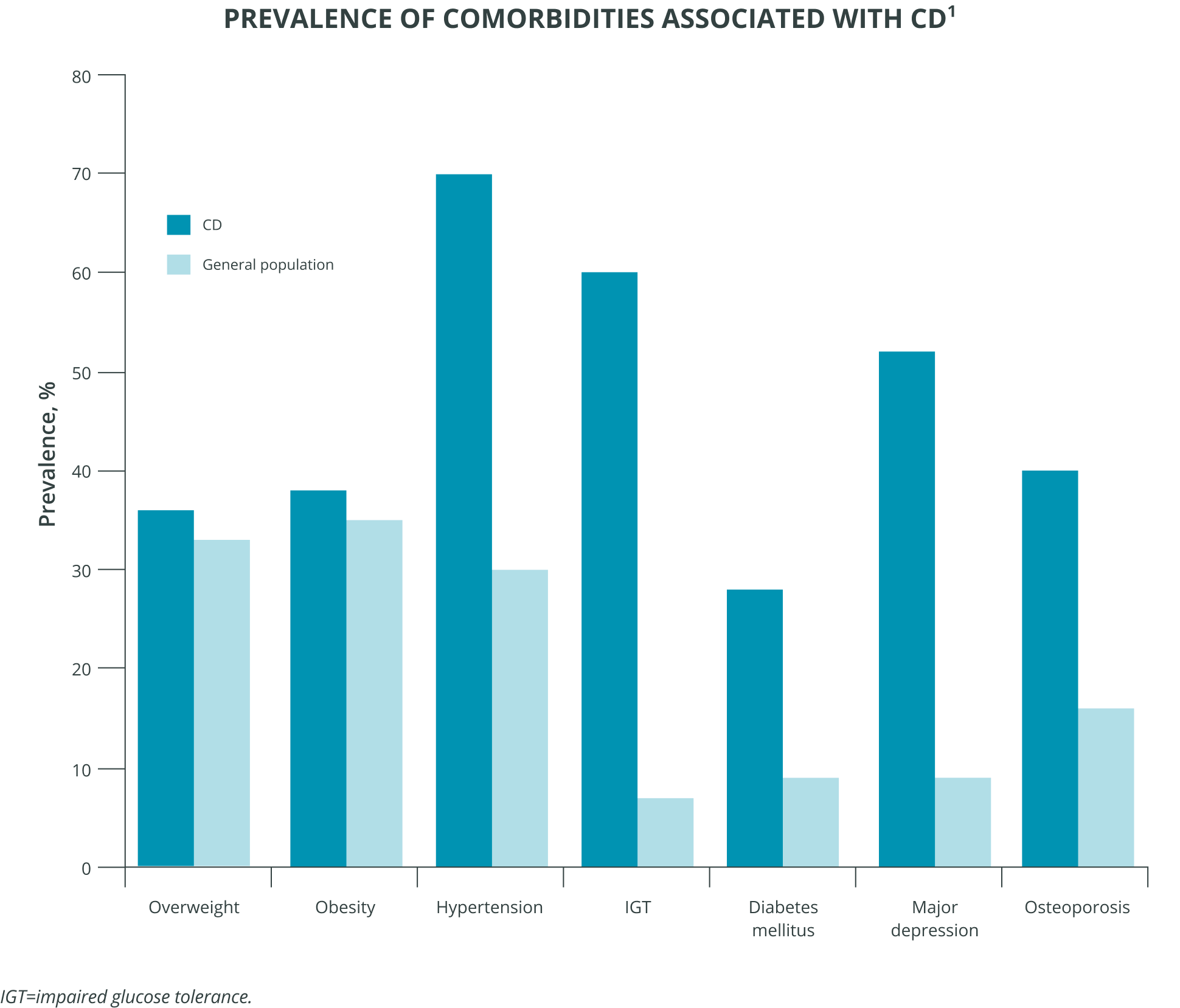
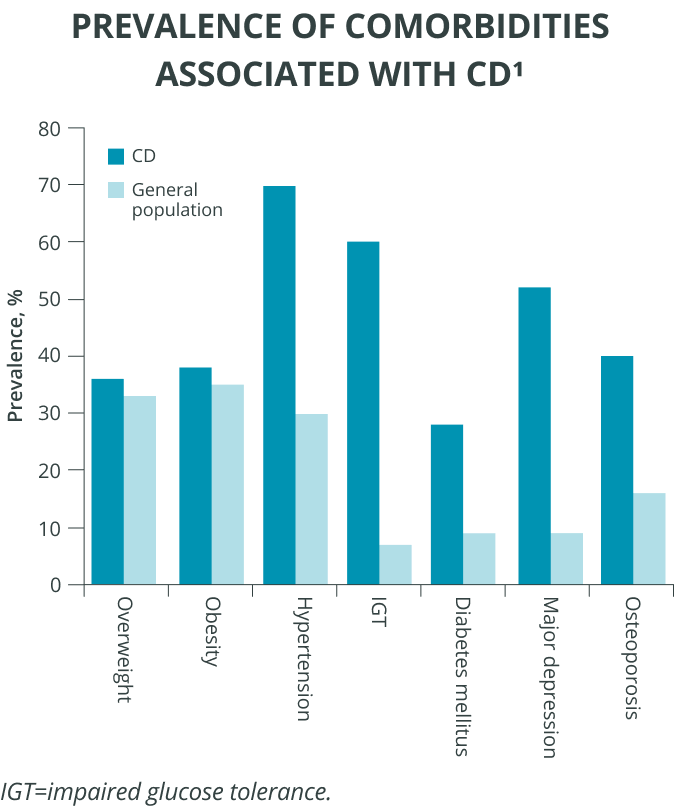
Hypercortisolism exposure poses risks
In fact, the longer the length of hypercortisolism exposure, the greater the mortality risk.1,7 Even transient exposure to excess cortisol is associated with increased mortality.7 So evaluating and treating the long-term negative effects of chronic hypercortisolism may be important to reduce morbidity, improve quality of life, and reduce the long-term mortality associated with Cushing’s disease.6
Uncontrolled chronic hypercortisolism also leads to elevated risks of multi-system morbidity and mortality.8,9 Patients with chronic, uncontrolled hypercortisolism have a ~3.5-5x higher mortality risk than in the general population.10 While the risk of all-cause morbidity and mortality decreases with remission, it’s not entirely eliminated.9
PATIENTS WITH CHRONIC EXPOSURE TO EXCESS CORTISOL
are at increased risk for all-cause morbidity and mortality11
VTE

Heart Failure

Stroke

AMI

INDICATIONS AND USAGE
ISTURISA (osilodrostat) is a cortisol synthesis inhibitor indicated for the treatment of adult patients with Cushing’s disease for whom pituitary surgery is not an option or has not been curative.
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
-
Hypocortisolism: ISTURISA lowers cortisol levels and can lead to hypocortisolism and sometimes life-threatening adrenal insufficiency. Lowering of cortisol can cause nausea, vomiting, fatigue, abdominal pain, loss of appetite, and dizziness. Significant lowering of serum cortisol may result in hypotension, abnormal electrolyte levels, and hypoglycemia.
Hypocortisolism can occur at any time during ISTURISA treatment. Evaluate patients for precipitating causes of hypocortisolism (infection, physical stress, etc). Monitor 24-hr urine free cortisol, serum or plasma cortisol, and patient’s signs and symptoms periodically during ISTURISA treatment.
Decrease or temporarily discontinue ISTURISA if urine free cortisol levels fall below the target range, there is a rapid decrease in cortisol levels, and/or patients report symptoms of hypocortisolism. Stop ISTURISA and administer exogenous glucocorticoid replacement therapy if serum or plasma cortisol levels are below target range and patients have symptoms of adrenal insufficiency. After ISTURISA discontinuation, cortisol suppression may persist beyond the 4-hour half-life of ISTURISA. Please see section 5.1 of full Prescribing Information.
Educate patients on the symptoms associated with hypocortisolism and advise them to contact a healthcare provider if they occur.
- QTc Prolongation: ISTURISA is associated with a dose-dependent QT interval prolongation which may cause cardiac arrhythmias. Perform an ECG to obtain a baseline QTc interval measurement prior to initiating therapy with ISTURISA and monitor for an effect on the QTc interval thereafter. Correct hypokalemia and/or hypomagnesemia prior to ISTURISA initiation and monitor periodically during treatment with ISTURISA. Use with caution in patients with risk factors for QT prolongation and consider more frequent ECG monitoring. Please see section 5.2 of full Prescribing Information.
- Elevations in Adrenal Hormone Precursors and Androgens: ISTURISA blocks cortisol synthesis and may increase circulating levels of cortisol and aldosterone precursors and androgens. This may activate mineralocorticoid receptors and cause hypokalemia, edema and hypertension. Hypokalemia should be corrected prior to initiating ISTURISA. Monitor patients treated with ISTURISA for hypokalemia, worsening of hypertension and edema. Inform patients of the symptoms associated with hyperandrogenism and advise them to contact a healthcare provider if they occur. Please see section 5.3 of full Prescribing Information.
Adverse Reactions
- Most common adverse reactions (incidence >20%) are adrenal insufficiency, fatigue, nausea, headache, and edema.
- To report SUSPECTED ADVERSE REACTIONS, contact Recordati Rare Diseases Inc. at 1-888-575-8344, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- CYP3A4 Inhibitor: Reduce the dose of ISTURISA by half with concomitant use of a strong CYP3A4 inhibitor.
- CYP3A4 and CYP2B6 Inducers: An increase of ISTURISA dosage may be needed if ISTURISA is used concomitantly with strong CYP3A4 and CYP2B6 inducers. A reduction in ISTURISA dosage may be needed if strong CYP3A4 and CYP2B6 inducers are discontinued while using ISTURISA.
Use in Specific Populations
- Lactation: Breastfeeding is not recommended during treatment with ISTURISA and for at least one week after treatment.
INDICATIONS AND USAGE
ISTURISA (osilodrostat) is a cortisol synthesis inhibitor indicated for the treatment of adult patients with Cushing’s disease for whom pituitary surgery is not an option or has not been curative.
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
-
Hypocortisolism: ISTURISA lowers cortisol levels and can lead to hypocortisolism and sometimes life-threatening adrenal insufficiency. Lowering of cortisol can cause nausea, vomiting, fatigue, abdominal pain, loss of appetite, and dizziness. Significant lowering of serum cortisol may result in hypotension, abnormal electrolyte levels, and hypoglycemia.
Hypocortisolism can occur at any time during ISTURISA treatment. Evaluate patients for precipitating causes of hypocortisolism (infection, physical stress, etc). Monitor 24-hr urine free cortisol, serum or plasma cortisol, and patient’s signs and symptoms periodically during ISTURISA treatment.
Decrease or temporarily discontinue ISTURISA if urine free cortisol levels fall below the target range, there is a rapid decrease in cortisol levels, and/or patients report symptoms of hypocortisolism. Stop ISTURISA and administer exogenous glucocorticoid replacement therapy if serum or plasma cortisol levels are below target range and patients have symptoms of adrenal insufficiency. After ISTURISA discontinuation, cortisol suppression may persist beyond the 4-hour half-life of ISTURISA. Please see section 5.1 of full Prescribing Information.
Educate patients on the symptoms associated with hypocortisolism and advise them to contact a healthcare provider if they occur.
- QTc Prolongation: ISTURISA is associated with a dose-dependent QT interval prolongation which may cause cardiac arrhythmias. Perform an ECG to obtain a baseline QTc interval measurement prior to initiating therapy with ISTURISA and monitor for an effect on the QTc interval thereafter. Correct hypokalemia and/or hypomagnesemia prior to ISTURISA initiation and monitor periodically during treatment with ISTURISA. Use with caution in patients with risk factors for QT prolongation and consider more frequent ECG monitoring. Please see section 5.2 of full Prescribing Information.
- Elevations in Adrenal Hormone Precursors and Androgens: ISTURISA blocks cortisol synthesis and may increase circulating levels of cortisol and aldosterone precursors and androgens. This may activate mineralocorticoid receptors and cause hypokalemia, edema and hypertension. Hypokalemia should be corrected prior to initiating ISTURISA. Monitor patients treated with ISTURISA for hypokalemia, worsening of hypertension and edema. Inform patients of the symptoms associated with hyperandrogenism and advise them to contact a healthcare provider if they occur. Please see section 5.3 of full Prescribing Information.
Adverse Reactions
- Most common adverse reactions (incidence >20%) are adrenal insufficiency, fatigue, nausea, headache, and edema.
- To report SUSPECTED ADVERSE REACTIONS, contact Recordati Rare Diseases Inc. at 1‑888‑575‑8344, or FDA at 1‑800‑FDA‑1088 or www.fda.gov/medwatch.
Drug Interactions
- CYP3A4 Inhibitor: Reduce the dose of ISTURISA by half with concomitant use of a strong CYP3A4 inhibitor.
- CYP3A4 and CYP2B6 Inducers: An increase of ISTURISA dosage may be needed if ISTURISA is used concomitantly with strong CYP3A4 and CYP2B6 inducers. A reduction in ISTURISA dosage may be needed if strong CYP3A4 and CYP2B6 inducers are discontinued while using ISTURISA.
Use in Specific Populations
- Lactation: Breastfeeding is not recommended during treatment with ISTURISA and for at least one week after treatment.
