You are now leaving the ISTURISA (osilodrostat) website. This link will take you to a site maintained by a third party who is solely responsible for its content.
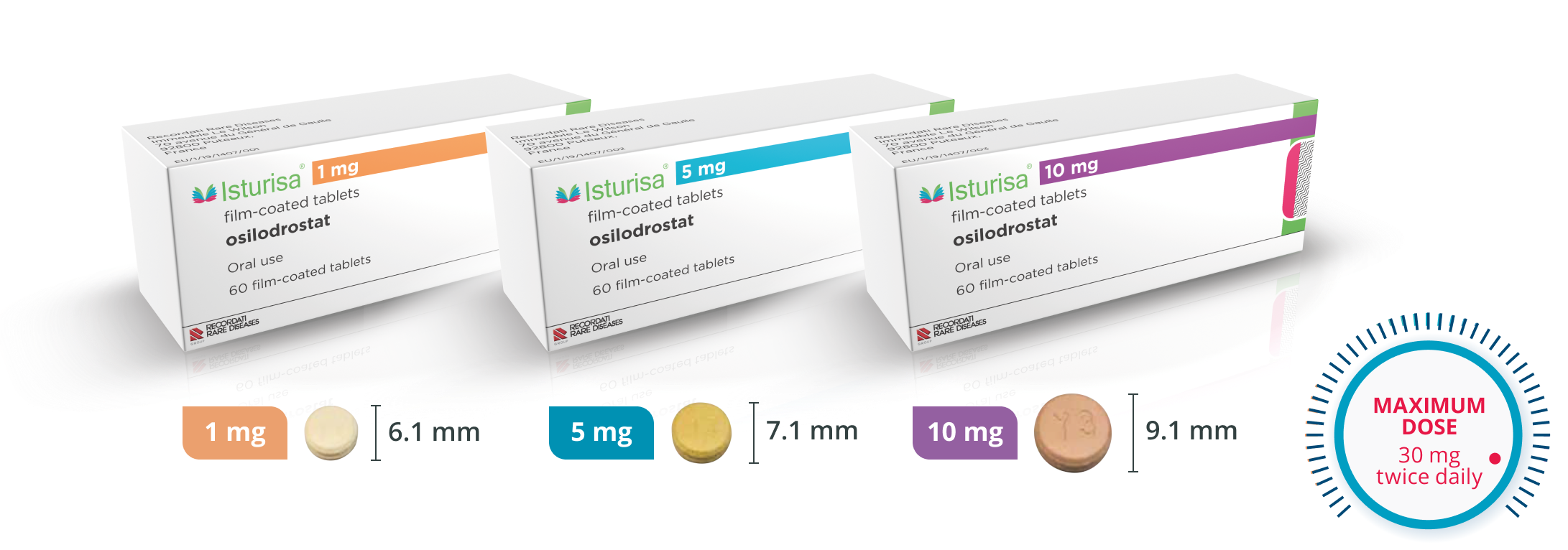
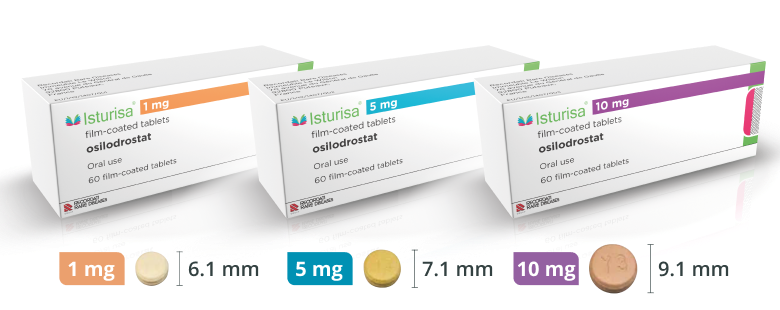
Multiple Dosing Options for Flexibility
Multiple dosing options allow you to adjust therapy for each patient’s unique needs.1


START
ISTURISA 2 mg twice daily with or without food.*

TITRATE
Up in 1- or 2-mg increments twice daily, no more than once every 2 weeks based on individual response and tolerability. If your patient tolerates 10 mg twice daily and continues to have elevated 24-hour UFC levels above the ULN, you may up-titrate by 5 mg twice daily every 2 weeks.

MONITOR
Cortisol levels every 1 to 2 weeks until adequate clinical response is maintained. Consider less frequent monitoring thereafter.
Recommended testing and monitoring1

What to test prior to starting ISTURISA
- Test magnesium and potassium levels
- Correct for hypokalemia and hypomagnesemia
- Obtain baseline ECG

Continue monitoring your patients to optimize titration
- Monitor cortisol levels from at least two 24-hour urine free cortisol collections every 1-2 weeks until adequate clinical response is maintained
- Repeat ECG within one week after treatment initiation, and as clinically indicated thereafter
- Monitor potassium and magnesium levels
- Monitor the signs and symptoms of hypercortisolism to help reduce life-threatening complications
Guidelines recommend urgent treatment of hypercortisolism (within 24-72 hours)2
TREAT IMMEDIATELY IF YOU SEE SIGNS OF ANY OF THE FOLLOWING:2

Infection

Acute
psychosis

Pulmonary thromboembolism

Cardiovascular complications
INDICATIONS AND USAGE
ISTURISA (osilodrostat) is a cortisol synthesis inhibitor indicated for the treatment of adult patients with Cushing’s disease for whom pituitary surgery is not an option or has not been curative.
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
-
Hypocortisolism: ISTURISA lowers cortisol levels and can lead to hypocortisolism and sometimes life-threatening adrenal insufficiency. Lowering of cortisol can cause nausea, vomiting, fatigue, abdominal pain, loss of appetite, and dizziness. Significant lowering of serum cortisol may result in hypotension, abnormal electrolyte levels, and hypoglycemia.
Hypocortisolism can occur at any time during ISTURISA treatment. Evaluate patients for precipitating causes of hypocortisolism (infection, physical stress, etc). Monitor 24-hr urine free cortisol, serum or plasma cortisol, and patient’s signs and symptoms periodically during ISTURISA treatment.
Decrease or temporarily discontinue ISTURISA if urine free cortisol levels fall below the target range, there is a rapid decrease in cortisol levels, and/or patients report symptoms of hypocortisolism. Stop ISTURISA and administer exogenous glucocorticoid replacement therapy if serum or plasma cortisol levels are below target range and patients have symptoms of adrenal insufficiency. After ISTURISA discontinuation, cortisol suppression may persist beyond the 4-hour half-life of ISTURISA. Please see section 5.1 of full Prescribing Information.
Educate patients on the symptoms associated with hypocortisolism and advise them to contact a healthcare provider if they occur.
- QTc Prolongation: ISTURISA is associated with a dose-dependent QT interval prolongation which may cause cardiac arrhythmias. Perform an ECG to obtain a baseline QTc interval measurement prior to initiating therapy with ISTURISA and monitor for an effect on the QTc interval thereafter. Correct hypokalemia and/or hypomagnesemia prior to ISTURISA initiation and monitor periodically during treatment with ISTURISA. Use with caution in patients with risk factors for QT prolongation and consider more frequent ECG monitoring. Please see section 5.2 of full Prescribing Information.
- Elevations in Adrenal Hormone Precursors and Androgens: ISTURISA blocks cortisol synthesis and may increase circulating levels of cortisol and aldosterone precursors and androgens. This may activate mineralocorticoid receptors and cause hypokalemia, edema and hypertension. Hypokalemia should be corrected prior to initiating ISTURISA. Monitor patients treated with ISTURISA for hypokalemia, worsening of hypertension and edema. Inform patients of the symptoms associated with hyperandrogenism and advise them to contact a healthcare provider if they occur. Please see section 5.3 of full Prescribing Information.
Adverse Reactions
- Most common adverse reactions (incidence >20%) are adrenal insufficiency, fatigue, nausea, headache, and edema.
- To report SUSPECTED ADVERSE REACTIONS, contact Recordati Rare Diseases Inc. at 1-888-575-8344, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- CYP3A4 Inhibitor: Reduce the dose of ISTURISA by half with concomitant use of a strong CYP3A4 inhibitor.
- CYP3A4 and CYP2B6 Inducers: An increase of ISTURISA dosage may be needed if ISTURISA is used concomitantly with strong CYP3A4 and CYP2B6 inducers. A reduction in ISTURISA dosage may be needed if strong CYP3A4 and CYP2B6 inducers are discontinued while using ISTURISA.
Use in Specific Populations
- Lactation: Breastfeeding is not recommended during treatment with ISTURISA and for at least one week after treatment.
INDICATIONS AND USAGE
ISTURISA (osilodrostat) is a cortisol synthesis inhibitor indicated for the treatment of adult patients with Cushing’s disease for whom pituitary surgery is not an option or has not been curative.
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
-
Hypocortisolism: ISTURISA lowers cortisol levels and can lead to hypocortisolism and sometimes life-threatening adrenal insufficiency. Lowering of cortisol can cause nausea, vomiting, fatigue, abdominal pain, loss of appetite, and dizziness. Significant lowering of serum cortisol may result in hypotension, abnormal electrolyte levels, and hypoglycemia.
Hypocortisolism can occur at any time during ISTURISA treatment. Evaluate patients for precipitating causes of hypocortisolism (infection, physical stress, etc). Monitor 24-hr urine free cortisol, serum or plasma cortisol, and patient’s signs and symptoms periodically during ISTURISA treatment.
Decrease or temporarily discontinue ISTURISA if urine free cortisol levels fall below the target range, there is a rapid decrease in cortisol levels, and/or patients report symptoms of hypocortisolism. Stop ISTURISA and administer exogenous glucocorticoid replacement therapy if serum or plasma cortisol levels are below target range and patients have symptoms of adrenal insufficiency. After ISTURISA discontinuation, cortisol suppression may persist beyond the 4-hour half-life of ISTURISA. Please see section 5.1 of full Prescribing Information.
Educate patients on the symptoms associated with hypocortisolism and advise them to contact a healthcare provider if they occur.
- QTc Prolongation: ISTURISA is associated with a dose-dependent QT interval prolongation which may cause cardiac arrhythmias. Perform an ECG to obtain a baseline QTc interval measurement prior to initiating therapy with ISTURISA and monitor for an effect on the QTc interval thereafter. Correct hypokalemia and/or hypomagnesemia prior to ISTURISA initiation and monitor periodically during treatment with ISTURISA. Use with caution in patients with risk factors for QT prolongation and consider more frequent ECG monitoring. Please see section 5.2 of full Prescribing Information.
- Elevations in Adrenal Hormone Precursors and Androgens: ISTURISA blocks cortisol synthesis and may increase circulating levels of cortisol and aldosterone precursors and androgens. This may activate mineralocorticoid receptors and cause hypokalemia, edema and hypertension. Hypokalemia should be corrected prior to initiating ISTURISA. Monitor patients treated with ISTURISA for hypokalemia, worsening of hypertension and edema. Inform patients of the symptoms associated with hyperandrogenism and advise them to contact a healthcare provider if they occur. Please see section 5.3 of full Prescribing Information.
Adverse Reactions
- Most common adverse reactions (incidence >20%) are adrenal insufficiency, fatigue, nausea, headache, and edema.
- To report SUSPECTED ADVERSE REACTIONS, contact Recordati Rare Diseases Inc. at 1‑888‑575‑8344, or FDA at 1‑800‑FDA‑1088 or www.fda.gov/medwatch.
Drug Interactions
- CYP3A4 Inhibitor: Reduce the dose of ISTURISA by half with concomitant use of a strong CYP3A4 inhibitor.
- CYP3A4 and CYP2B6 Inducers: An increase of ISTURISA dosage may be needed if ISTURISA is used concomitantly with strong CYP3A4 and CYP2B6 inducers. A reduction in ISTURISA dosage may be needed if strong CYP3A4 and CYP2B6 inducers are discontinued while using ISTURISA.
Use in Specific Populations
- Lactation: Breastfeeding is not recommended during treatment with ISTURISA and for at least one week after treatment.
