You are now leaving the ISTURISA (osilodrostat) website. This link will take you to a site maintained by a third party who is solely responsible for its content.
Clinical Studies
ISTURISA efficacy was assessed in two Phase 3 clinical studies. Select a study to review efficacy data.
LINC 3 is a rigorous phase III clinical trial (N=137 patients with CD) that included a randomized, double-blind, placebo-controlled withdrawal period.1,2
Inclusion criteria2
- Patients aged 18–75 years with a confirmed diagnosis of persistent, recurrent, or de novo (if not surgical candidates) Cushing’s disease
- Confirmed Cushing’s disease as determined by mUFC >1.5 x ULN at screening
- Patients with a history of pituitary surgery had to be at least 30 days post surgery
Baseline characteristics1,2
- Mean age at enrollment was 41 years
- 77% of patients were female
- 88% of enrolled patients had previous surgery
- Mean mUFC at baseline was 365 μg/24 hr (~7 x ULN)
- Median mUFC was 173 μg/24 hr (~3.5 x ULN)

 Titration occurred every 2 weeks if mUFC was greater than the ULN.1
Titration occurred every 2 weeks if mUFC was greater than the ULN.1
ISTURISA effectively normalized and sustained mUFC in the majority of patients and demonstrated superiority over placebo at maintaining mUFC ≤ULN at the end of period 3 randomized withdrawal (n=71, P<0.001).1
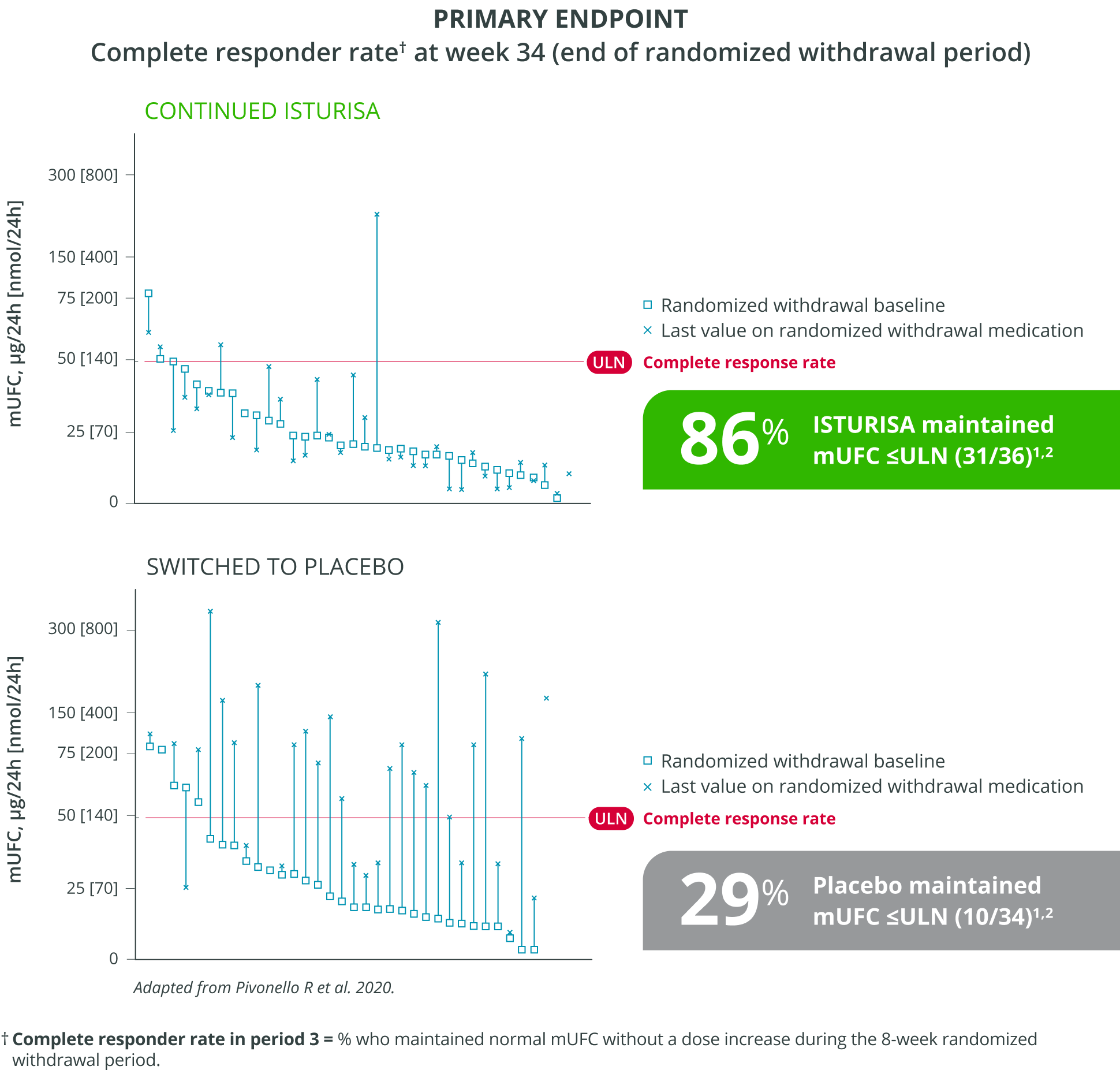
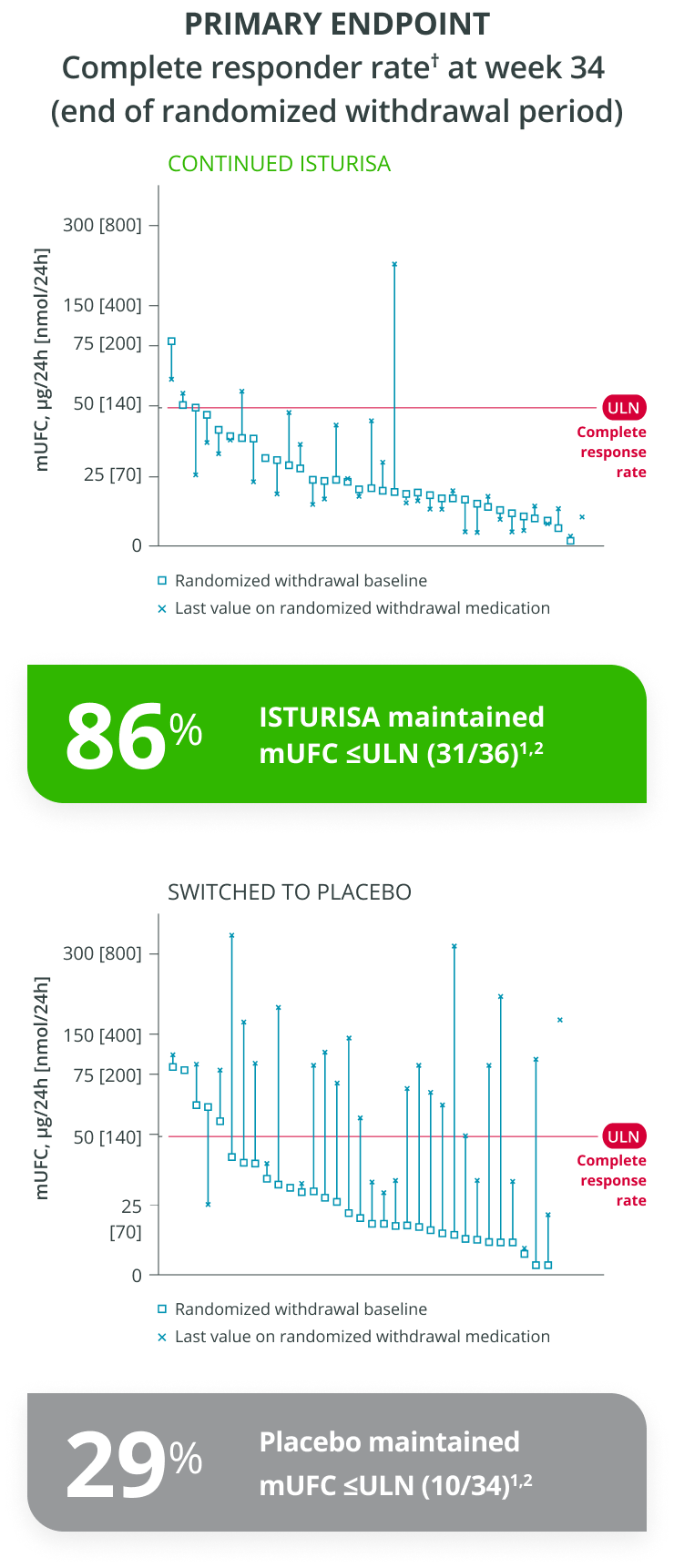 Adapted from Pivonello R et al. 2020.
Complete responder rate in period 3 = % who maintained normal mUFC without a dose increase during the 8-week randomized withdrawal period.
Adapted from Pivonello R et al. 2020.
Complete responder rate in period 3 = % who maintained normal mUFC without a dose increase during the 8-week randomized withdrawal period.
Key data demonstrated early improvements with ISTURISA. A significant portion of patients maintained normalized mUFC with ISTURISA at the therapeutic dose established during the titration period.1,2
Key secondary ENDPOINT
Complete responder rate at week 24 (end of maintenance period)
Additional data
Demonstrated other signs of improvement with ISTURISA2,§

Irrespective of dose increase, the percentage of patients who achieved mUFC <ULN at week 24 (N=93/137) was
68%
The median
time to first
complete response
(mUFC ≤ULN)
was

The percentage of patients who achieved cortisol normalization at least once during the study was
96%ISTURISA sustained normal mUFC up to 48 weeks in the majority of patients.1 Mean mUFC decreased rapidly during the titration period, then remained below baseline through week 48.3
48-WEEK STUDY ENDPOINT
(ITT analysis, end of open-label period)
MEAN (±SEM) mUFC AT TIME POINTS UP TO WEEK 48,
among all patients who received treatment in the randomized withdrawal period3
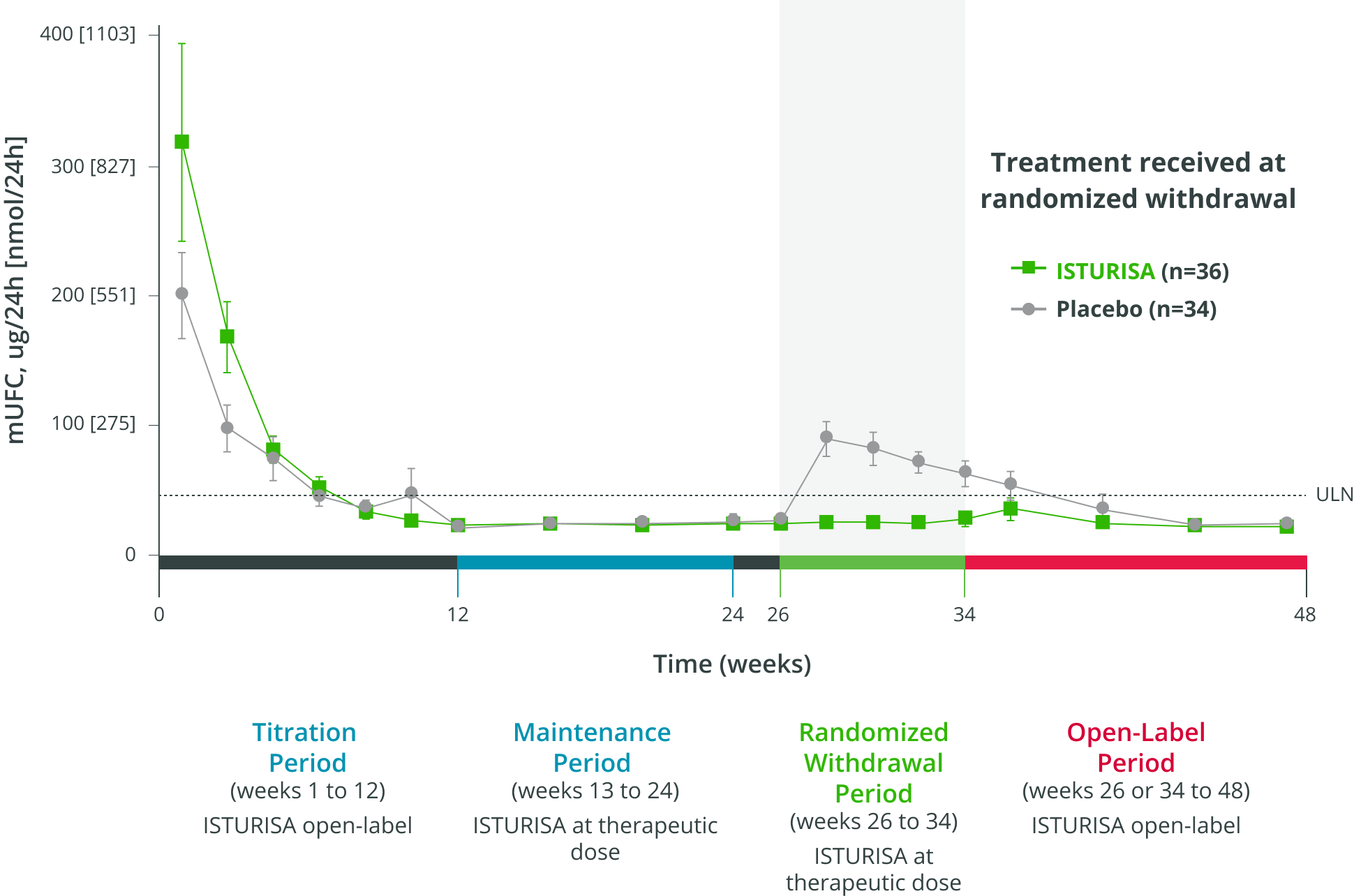
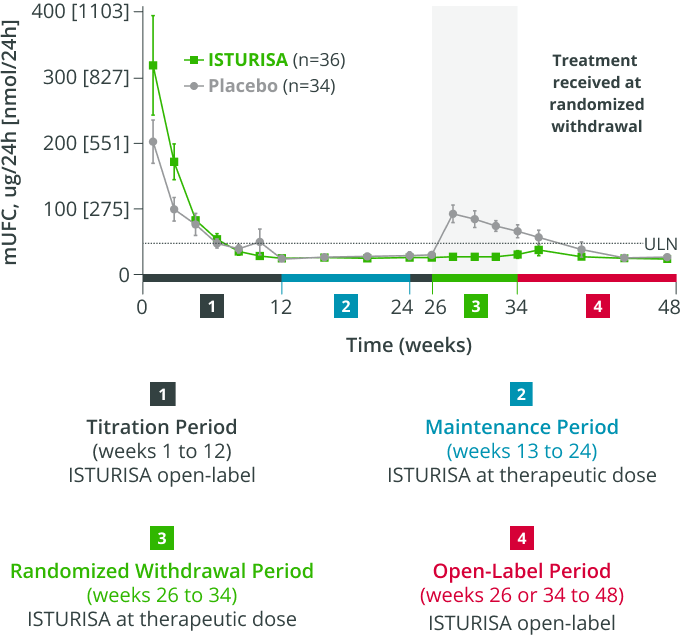
- Average daily ISTURISA dose for all patients through 48 weeks was 10 mg/day4,||
Improvements were maintained for over a year in the majority of patients who entered the 72-week open-label extension (N=106).5
LINC 3 EXTENSION RESULTS
Complete responder rate at week 72
Improvements in cardiovascular-related parameters
were maintained through the open-label extension5
 Systolic blood pressure (mmHg)
Systolic blood pressure (mmHg)
 Diastolic blood pressure (mmHg)
Diastolic blood pressure (mmHg)
 HbA1c (%)
HbA1c (%)
 BMI (kg/m2)
BMI (kg/m2)
ISTURISA was well tolerated, with no new safety signals reported during long-term treatment5
- 106 of 137 enrolled patients entered the optional extension5
- Median duration of ISTURISA treatment from baseline to study end for all enrolled patients was 130 weeks with a median dose of 7.4 mg/day5
- Most hypocortisolism-related AEs emerged during the first 26 weeks of treatment and AEs related to adrenal hormone precursor accumulation were less frequent in the extension5
- Mean testosterone levels in women increased upon initiation of ISTURISA but tended to return to baseline levels and within the normal range at 72 weeks (0.8 × ULN)1,5
Improvements were also observed for metabolic, CushingQoL, and depression parameters.1,2 Overall, mean improvements were observed from baseline.2,4
 Systolic blood pressure (mmHg)¶
Systolic blood pressure (mmHg)¶
 Diastolic blood pressure (mmHg)¶
Diastolic blood pressure (mmHg)¶
 Body weight (kg)
Body weight (kg)
 Waist circumference (cm)
Waist circumference (cm)
 HbA1c (%)¶
HbA1c (%)¶
 Total cholesterol (mg/dL)
Total cholesterol (mg/dL)
 CushingQoL (total score)#
CushingQoL (total score)#
 BDI-II (total score)**
BDI-II (total score)**
Because the study allowed initiation of anti-hypertensive and anti-diabetic medications and dose increases in patients already receiving such medications and there was absence of a control group, the individual contribution of ISTURISA or of anti-hypertensive and anti-diabetic medication adjustments cannot be clearly established.
CushingQoL is a disease-generated questionnaire proven to be a reliable and valid instrument for measuring health-related quality of life in patients with Cushing’s syndrome. Scores correlate with relevant clinical parameters.6 BDI-II is a proven valid and reliable 21-item self-report multiple-choice inventory tool used to evaluate depressive states that occur at high prevalence among the medically ill.7 BDI-II, Beck Depression Inventory-II; CushingQoL, Cushing’s Quality of Life questionnaire; HbA1c, glycosylated hemoglobin.LINC 4 is a randomized, double-blind, parallel, placebo-controlled clinical trial that evaluated the safety and efficacy of ISTURISA among 73 patients with CD (mUFC >1.3 × ULN).8
Key learnings from LINC 4 study:8

ISTURISA provides rapid and sustained normalization of cortisol

ISTURISA is well tolerated and has a manageable safety profile

Response and tolerability may be managed with closing monitoring and dose adjustments

 Patients started on ISTURISA 2 mg twice daily (baseline median mUFC was 2.5 × ULN [0.7-12.5]) or matching placebo (baseline median mUFC was 2.2 × ULN [0.2-18.9]). Dose adjustments to normalize mUFC or to address safety issues were permitted at week 2, week 5, and week 8 (range 1-20 mg bid) based on efficacy and tolerability.8
Starting at week 12, all patients received open-label ISTURISA, with dose adjustments permitted (range 1–30 mg bid).8
Patients started on ISTURISA 2 mg twice daily (baseline median mUFC was 2.5 × ULN [0.7-12.5]) or matching placebo (baseline median mUFC was 2.2 × ULN [0.2-18.9]). Dose adjustments to normalize mUFC or to address safety issues were permitted at week 2, week 5, and week 8 (range 1-20 mg bid) based on efficacy and tolerability.8
Starting at week 12, all patients received open-label ISTURISA, with dose adjustments permitted (range 1–30 mg bid).8
ISTURISA rapidly and effectively normalized cortisol levels in the majority of patients, and demonstrated superiority over placebo at normalizing cortisol (mUFC ≤ULN) at week 12 (N=73, P<0.0001).8
PRIMARY ENDPOINT
Significantly more patients had mUFC ≤ULN with ISTURISA at week 12 (P<0.0001)8
12-week, double-blind, parallel, placebo-controlled period (N=73)
(OR 43.4;
95% CI 7.1-343.2)
Dose adjustments were made at weeks 2, 5, and 8 followed by adjustments every 3 weeks based on patient response and tolerability8,*
Note: Adverse events related to hypocortisolism occurred in 15% vs 0% of ISTURISA and placebo patients during titration.8
Patients started on ISTURISA 2 mg twice daily (baseline median mUFC was 2.5 × ULN [0.7-12.5]) or matching placebo (baseline median mUFC was 2.2 × ULN [0.2-18.9]). Dose adjustments to normalize mUFC or to address safety issues were permitted at week 2, week 5, and week 8 (range 1-20 mg bid) based on effi cacy and tolerability.8ISTURISA effectively sustained cortisol normalization for more than 4 out of 5 patients at week 36 in the open-label period (N=73).8
Key secondary ENDPOINT
Proportion of patients with mUFC ≤ULN at week 36
Median ISTURISA dose at study end was8
- 4.7 mg/day in patients initially assigned to ISTURISA
- 6.0 mg/day in patients initially assigned to placebo
ISTURISA titration was slower in LINC 4 without compromising the ability to achieve cortisol normalization.9
Patients in LINC 3
received dose increases every
2 weeks9
Patients in LINC 4
received dose increases approximately every
3 weeks9
Recent Advances in Cushing’s Disease
Highlights from a clinical case study and the use of ISTURISA.
Watch video on-demandINDICATIONS AND USAGE
ISTURISA (osilodrostat) is a cortisol synthesis inhibitor indicated for the treatment of adult patients with Cushing’s disease for whom pituitary surgery is not an option or has not been curative.
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
-
Hypocortisolism: ISTURISA lowers cortisol levels and can lead to hypocortisolism and sometimes life-threatening adrenal insufficiency. Lowering of cortisol can cause nausea, vomiting, fatigue, abdominal pain, loss of appetite, and dizziness. Significant lowering of serum cortisol may result in hypotension, abnormal electrolyte levels, and hypoglycemia.
Hypocortisolism can occur at any time during ISTURISA treatment. Evaluate patients for precipitating causes of hypocortisolism (infection, physical stress, etc). Monitor 24-hr urine free cortisol, serum or plasma cortisol, and patient’s signs and symptoms periodically during ISTURISA treatment.
Decrease or temporarily discontinue ISTURISA if urine free cortisol levels fall below the target range, there is a rapid decrease in cortisol levels, and/or patients report symptoms of hypocortisolism. Stop ISTURISA and administer exogenous glucocorticoid replacement therapy if serum or plasma cortisol levels are below target range and patients have symptoms of adrenal insufficiency. After ISTURISA discontinuation, cortisol suppression may persist beyond the 4-hour half-life of ISTURISA. Please see section 5.1 of full Prescribing Information.
Educate patients on the symptoms associated with hypocortisolism and advise them to contact a healthcare provider if they occur.
- QTc Prolongation: ISTURISA is associated with a dose-dependent QT interval prolongation which may cause cardiac arrhythmias. Perform an ECG to obtain a baseline QTc interval measurement prior to initiating therapy with ISTURISA and monitor for an effect on the QTc interval thereafter. Correct hypokalemia and/or hypomagnesemia prior to ISTURISA initiation and monitor periodically during treatment with ISTURISA. Use with caution in patients with risk factors for QT prolongation and consider more frequent ECG monitoring. Please see section 5.2 of full Prescribing Information.
- Elevations in Adrenal Hormone Precursors and Androgens: ISTURISA blocks cortisol synthesis and may increase circulating levels of cortisol and aldosterone precursors and androgens. This may activate mineralocorticoid receptors and cause hypokalemia, edema and hypertension. Hypokalemia should be corrected prior to initiating ISTURISA. Monitor patients treated with ISTURISA for hypokalemia, worsening of hypertension and edema. Inform patients of the symptoms associated with hyperandrogenism and advise them to contact a healthcare provider if they occur. Please see section 5.3 of full Prescribing Information.
Adverse Reactions
- Most common adverse reactions (incidence >20%) are adrenal insufficiency, fatigue, nausea, headache, and edema.
- To report SUSPECTED ADVERSE REACTIONS, contact Recordati Rare Diseases Inc. at 1-888-575-8344, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- CYP3A4 Inhibitor: Reduce the dose of ISTURISA by half with concomitant use of a strong CYP3A4 inhibitor.
- CYP3A4 and CYP2B6 Inducers: An increase of ISTURISA dosage may be needed if ISTURISA is used concomitantly with strong CYP3A4 and CYP2B6 inducers. A reduction in ISTURISA dosage may be needed if strong CYP3A4 and CYP2B6 inducers are discontinued while using ISTURISA.
Use in Specific Populations
- Lactation: Breastfeeding is not recommended during treatment with ISTURISA and for at least one week after treatment.
INDICATIONS AND USAGE
ISTURISA (osilodrostat) is a cortisol synthesis inhibitor indicated for the treatment of adult patients with Cushing’s disease for whom pituitary surgery is not an option or has not been curative.
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
-
Hypocortisolism: ISTURISA lowers cortisol levels and can lead to hypocortisolism and sometimes life-threatening adrenal insufficiency. Lowering of cortisol can cause nausea, vomiting, fatigue, abdominal pain, loss of appetite, and dizziness. Significant lowering of serum cortisol may result in hypotension, abnormal electrolyte levels, and hypoglycemia.
Hypocortisolism can occur at any time during ISTURISA treatment. Evaluate patients for precipitating causes of hypocortisolism (infection, physical stress, etc). Monitor 24-hr urine free cortisol, serum or plasma cortisol, and patient’s signs and symptoms periodically during ISTURISA treatment.
Decrease or temporarily discontinue ISTURISA if urine free cortisol levels fall below the target range, there is a rapid decrease in cortisol levels, and/or patients report symptoms of hypocortisolism. Stop ISTURISA and administer exogenous glucocorticoid replacement therapy if serum or plasma cortisol levels are below target range and patients have symptoms of adrenal insufficiency. After ISTURISA discontinuation, cortisol suppression may persist beyond the 4-hour half-life of ISTURISA. Please see section 5.1 of full Prescribing Information.
Educate patients on the symptoms associated with hypocortisolism and advise them to contact a healthcare provider if they occur.
- QTc Prolongation: ISTURISA is associated with a dose-dependent QT interval prolongation which may cause cardiac arrhythmias. Perform an ECG to obtain a baseline QTc interval measurement prior to initiating therapy with ISTURISA and monitor for an effect on the QTc interval thereafter. Correct hypokalemia and/or hypomagnesemia prior to ISTURISA initiation and monitor periodically during treatment with ISTURISA. Use with caution in patients with risk factors for QT prolongation and consider more frequent ECG monitoring. Please see section 5.2 of full Prescribing Information.
- Elevations in Adrenal Hormone Precursors and Androgens: ISTURISA blocks cortisol synthesis and may increase circulating levels of cortisol and aldosterone precursors and androgens. This may activate mineralocorticoid receptors and cause hypokalemia, edema and hypertension. Hypokalemia should be corrected prior to initiating ISTURISA. Monitor patients treated with ISTURISA for hypokalemia, worsening of hypertension and edema. Inform patients of the symptoms associated with hyperandrogenism and advise them to contact a healthcare provider if they occur. Please see section 5.3 of full Prescribing Information.
Adverse Reactions
- Most common adverse reactions (incidence >20%) are adrenal insufficiency, fatigue, nausea, headache, and edema.
- To report SUSPECTED ADVERSE REACTIONS, contact Recordati Rare Diseases Inc. at 1‑888‑575‑8344, or FDA at 1‑800‑FDA‑1088 or www.fda.gov/medwatch.
Drug Interactions
- CYP3A4 Inhibitor: Reduce the dose of ISTURISA by half with concomitant use of a strong CYP3A4 inhibitor.
- CYP3A4 and CYP2B6 Inducers: An increase of ISTURISA dosage may be needed if ISTURISA is used concomitantly with strong CYP3A4 and CYP2B6 inducers. A reduction in ISTURISA dosage may be needed if strong CYP3A4 and CYP2B6 inducers are discontinued while using ISTURISA.
Use in Specific Populations
- Lactation: Breastfeeding is not recommended during treatment with ISTURISA and for at least one week after treatment.
